This study caught my eye caught my eye because the lead author is Stephen Smith, the author of one of the best ECG blogs out there. He also writes for other blogs, and at least one EMS ECG blog follows his work. Dr Smith is, in short, the man.
The study also caught my eye because it uses a retooled version on the ResQPOD, now called the ResQGARD. I know a lot of EMS folk swear by the ResQPOD, but the recent evidence has not proven its value. So, it's interesting to see "part 2" of the ResQPOD saga.
 |
| Because Part 1 worked out so well. |
The ResQGARD works on the same general physiologic principle as the ResQPOD. It allows for normal, unimpeded exhalation, and does not provide any PEEP. During inhalation, however, it slightly increases the force required to draw in a breath. The actively expanding thorax normally acts as a sort of "suction" to also pull blood up from the belly, but with this added resistance to the air inflow, this "suction" effect is magnified.
And there is some animal and human data to back up the claims for usefulness in treating hypotension.
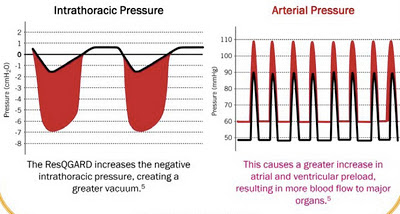 |
| Red means more blood, I guess. |
This device is evidently "cleared" by the FDA for treating "low blood circulation," and various studies have shown an ability to raise the blood pressure in, for example, blood-donors, or in other models of hypovolemia or hemorrhage. You can check out some background device in this article from Journal of Special Operations Medicine, the coolest journal that you aren't reading yet.
 |
| "Oh, is that JEMS you're reading? That's cute." |
This trial had two parts. In the first part, the device was tested in a randomized, controlled, and blinded fashion in the emergency department for patients with hypotension due to various causes. The primary endpoint was the maximum change in SBP over the first 10 minutes after placement of the device. They enrolled 47 hypotensive patients. These patients ended up with diagnoses of dehydration, sepsis, or hemorrhage most of the time. You can see the average change in SBP in the table below, broken down by the cause of the hypotension.
When they looked at the overall results, they found that patients who had the real device had an average rise in SBP of 12.9 mmHg, while patients who got the sham device only had a rise of 5.9 mmHg, a difference of 7 mmHg. They tell us that the difference is statistically significant.
In the second part, the device was used by EMS, with no control therapy, in an unblinded manner. It's not much of a study, and they were really only looking at "feasability" of using the device by prehospital providers. They also had 47 patients in this arm of the study, and they determined that, yes, it was feasible to use, and that patients tolerated the device well.
It's hard to interpret the data on the change in blood pressure, etc., in part 2, since, as we saw in the results of the first part, the average blood pressure tended to go up with or without the device. The bar graph below shows that the pressure came up to a statistically significant degree, but it can't tell us if this was better than doin' nuthin'.
So, what can we take from this paper?
In the end, not much. Let me list the reasons why:
- The difference in SBP is statistically significant, but unclear if clinically significant.
- Some of the causes for hypotension have established, beneficial treatments.
- Some of the causes require no treatment, and improve on their own.
- The majority of the literature supporting the use of this device is written, in part, by the inventor of the device.
In another article about the ResQGARD (or ITD-7) written by Smith, the authors present this table:
 |
| "Treatable" ≠ "Proven effective for" |
Let's look at the causes they list. First off, heat stroke is about heat load and mental status - the primary treatment is cooling, and hypovolemia is usually not a significant component of the problem.
The proper treatment of true dehydration is, well, hydration. As for sepsis, while hypotension is a manifestation of the problem, there is a large amount of evidence that large amounts of IV fluids, delivered rapidly, saves lives. Simply raising the pressure through other means is not appropriate.
Regarding hemorrhage, there is a thicket of controversy about optimum treatment. While much of current practice emphasizes normalizing the blood pressure, a lot of evidence suggests that "permissive hypotension" may be the best (non-)treatment. Where the ResQGARD falls in this area is not clear at all.
Lastly, orthostatic hypotension is usually transient, and requires no intensive therapy; e.g. juice & cookies after blood donation.
Let me speak of the appearance of bias in the studies supporting the ResQGARD. The inventor of the device is Keith Lurie, a cardiologist. I have no doubt that he has aspirations to advance medical science and save lives. Unfortunately, as the inventor of the device in question, and the owner of the company that sells them, he has a vested interest in selling the device. And, while it's not the most expensive medical device out there, it costs real money.
Look at the references listed in the paper. Of the 23 studies that Smith et al. provide as references, 20 had Dr. Lurie as a co-author. That's a real conflict of interest. By way of example, check out this intervew that appeared with Dr. Lurie in an EMS blog. He had an interesting take in the failure of the ResQPOD to show an effect in the ROC trial.
Interviewer: "I think many of us who have been following the ResQPOD were surprised by the recent announcement by the National Institute of Health that the ROC PRIMED trial was stopping enrollment. ... Considering that the ROC PRIMED trial was a prospective, multi-centered, randomized clinical trial with large enrollment, are you concerned about these results?
Dr. Lurie: "To directly answer your question, I am not concerned with the results, nor am I surprised."







We had the ResQPod until sometime in 2010 when that study came out saying that they didn't improve survival. We were told that they would be phased out, but we should use the ones we had.
ReplyDeleteWe did, but not for patient care. We found that by turning on the LED light and rolling them into dark corners we could cause all sorts of mischief.
I heard a rumor that one guy randomly rolled them under police cars in bad neighborhoods. Just a rumor of course.
I was infected with genital herpes in 2017 and I have been on the quest for a cure right from the day I discovered I had the infection. October last year when I almost gave up, I stumbled upon a post on the internet where I copied the email of a man named Dr ogbekhilu who provides herbal solutions to diseases. i contacted him and got to an agreement with him and he sent me herbal remedy through the united parcel service alongside instruction on how to use it. To my greatest surprise, I got healed after 18days of using the remedy as assured by the doctor.I decided to share this because I know that there are lots of people out there that need help. contact the doctor via his website https://drogbekhiluherbalhome.simdif.com Contact him for remedy for infection like{1}HIV And AIDS{2}Diabetes{3}Epilepsy{4} Blood Cancer{5} HPV{6} ALS{7}herpes{8}kidney failure
Deletewhatsapp contact: +2348102460821
I was infected with genital herpes in 2017 and I have been on the quest for a cure right from the day I discovered I had the infection. October last year when I almost gave up, I stumbled upon a post on the internet where I copied the email of a man named Dr ogbekhilu who provides herbal solutions to diseases. i contacted him and got to an agreement with him and he sent me herbal remedy through the united parcel service alongside instruction on how to use it. To my greatest surprise, I got healed after 18days of using the remedy as assured by the doctor.I decided to share this because I know that there are lots of people out there that need help. contact the doctor via his website https://drogbekhiluherbalhome.simdif.com Contact him for remedy for infection like{1}HIV And AIDS{2}Diabetes{3}Epilepsy{4} Blood Cancer{5} HPV{6} ALS{7}herpes{8}kidney failure
Deletewhatsapp contact: +2348102460821
.
I was infected with genital herpes in 2017 and I have been on the quest for a cure right from the day I discovered I had the infection. October last year when I almost gave up, I stumbled upon a post on the internet where I copied the email of a man named Dr ogbekhilu who provides herbal solutions to diseases. i contacted him and got to an agreement with him and he sent me herbal remedy through the united parcel service alongside instruction on how to use it. To my greatest surprise, I got healed after 18days of using the remedy as assured by the doctor.I decided to share this because I know that there are lots of people out there that need help. contact the doctor via his website https://drogbekhiluherbalhome.simdif.com Contact him for remedy for infection like{1}HIV And AIDS{2}Diabetes{3}Epilepsy{4} Blood Cancer{5} HPV{6} ALS{7}herpes{8}kidney failure
Deletewhatsapp contact: +2348102460821
.
I Want To Appreciate Dr.OYAGU for his great deeds, I Was Diagnosed With type 2 Herpes Virus Last year,And i Was Looking For Solution To Be Cured Luckily I Saw Testimonies On How Dr.OYAGU Cure Herpes Virus I Decided To Contact Dr.OYAGU I Contacted Him He Prepared A Herbal Medicine Portion And Sent It To Me,I Started The Herbal Medicine For My Health.He Gave Me Step By Step Instructions On How To Apply It, When I Applied It As Instructed, I Was Cured Of This Deadly Herpes Within 2 weeks, I Am Now Herpes Negative.My Brother And Sister I No That There Are So Many People That Have The Same Herpes Virus Please contact Dr OYAGU To Help You Too,And Help Me To Thank Dr.OYAGU For Cure Me, I’m Cured By Dr. OYAGU Herbal Medicine,His Contact Email:oyaguherbalhome@gmail.com
ReplyDeleteOr Cell Whatsapp Number +2348101755322 thank you
I was infected with genital herpes in 2017 and I have been on the quest for a cure right from the day I discovered I had the infection. October last year when I almost gave up, I stumbled upon a post on the internet where I copied the email of a man named Dr ogbekhilu who provides herbal solutions to diseases. i contacted him and got to an agreement with him and he sent me herbal remedy through the united parcel service alongside instruction on how to use it. To my greatest surprise, I got healed after 18days of using the remedy as assured by the doctor.I decided to share this because I know that there are lots of people out there that need help. contact the doctor via his website https://drogbekhiluherbalhome.simdif.com Contact him for remedy for infection like{1}HIV And AIDS{2}Diabetes{3}Epilepsy{4} Blood Cancer{5} HPV{6} ALS{7}herpes{8}kidney failure
ReplyDeletewhatsapp contact: +2348102460821
.
I can’t believe this is really true I never believe there is cure to this hsv 2 because all the hospital have told me there is no cure to it, few months ago I saw this man email DR.AZIEGBE on internet from a testimony share by someone who he help with his herbal cure I contact his email and ask for his help also, that is how he inform me about the cure process and this man sent me a herbal medicine which I took according to the way he instructed for 2 week I can’t believe when I go for test my result come out negative i am so happy to share this to the world there is real cure to herpes you can also contact DR.AZIEGBE through his email now DRAZIEGBE1SPELLHOME@GMAIL. COM and also WhatsApp him +2349035465208. or https://draziegbeherbalhome.webs.com/ my assistant my email: JAMESAVA0001@GMAIL. COM... He also have herbs medicine to cured the following diseases;
ReplyDeleteDiabetes, Lupus, HPV, Gout, Hepatitis A,B, Infertility, HIV/AIDS, CANCER, WART
https://web.facebook.com/Herpes-std-cure-dr-aziegbe-herbal-cure-103360314788997/
HELLO everyone! Still don’t know the right words to express my Gratitude to the Great DR.Imoudu After been diagnosed with the #herpes for the passed 1 years, i was given so many health prescription and advice with no improvement, I totally lost hope, until i found many testimonies of Great DR.Imoudu in an online research Like anybody would be, i advice anyone that is living with herpes can also contact him today, because he has the cure to any virus problem contact him on WhatsApp +2348109609753..
ReplyDeleteOr his Email: DR.IMOUDUHEALERTEMPLE@GMAIL. COM
1...ALS CURE/DIABETES CURE/EPILESY/HPV CURE/LOOSE WEIGHT AND BODY/EX-BACK/HEPATITIS/
THANK YOU DR.Imoudu FOR YOU GOOD WORK IN MY LIFE..
I started on COPD Herbal treatment from Ultimate Health Home, the treatment worked incredibly for my lungs condition. I used the herbal treatment for almost 4 months, it reversed my COPD. My severe shortness of breath, dry cough, chest tightness gradually disappeared. Reach Ultimate Health Home via their website www.ultimatelifeclinic.com I can breath much better and It feels comfortable!
ReplyDelete
ReplyDeleteI was diagnosed with COPD four years ago and struggled with worsening symptoms despite using inhalers and medications. Last year, I tried a herbal treatment from NaturePath Herbal Clinic, and to my surprise, it made a huge difference. My breathing improved, the coughing eased, and my energy came back. I feel better than I have in years. If you're dealing with COPD, I highly recommend checking them out: www.naturepathherbalclinic.com.